2 billion years ago, nature created its own nuclear reactor: This is how it works!

"This can't be possible."
That was the thought that crossed the mind of physicist Francis Perrin in May 1972. Perrin was examining a dark lump of uranium ore at a nuclear fuel processing plant in southern France. This sample, extracted from a mine in Gabon, Africa, held a secret that challenged everything scientists knew about natural uranium.
Normally, uranium is composed of a consistent ratio of isotopes such as uranium-238, uranium-234, and the crucial uranium-235. The proportion of uranium-235 throughout the Earth's crust is consistent at 0.720 percent. However, this sample from Gabon contained only 0.717 percent uranium-235. While a small deviation, it was enough to raise alarms. The simplest explanation was that the uranium had undergone fission. But since this was a natural sample, how could this have happened?
Had someone been tampering with the uranium? Was this the work of an ancient, unknown civilization? Or perhaps something even stranger was at play?
Fission in nature
As scientists continued their investigations, the situation grew more complex. Some uranium samples from the Oklo region of Gabon contained even lower uranium-235 levels, as low as 0.4 percent . This was more than just a statistical fluke; it indicated a fundamental change in the ore. Further analysis revealed that the uranium had indeed undergone fission, the same process used in nuclear reactors. However, this was neither the result of human intervention nor an alien species. The evidence pointed to an event that had occurred two billion years ago. Suddenly, the unthinkable emerged: Nature had created its own nuclear reactor. France had mined uranium from Gabon, its colony at the time, for nearly 40 years. France was a major nuclear power, using uranium to generate electricity both at home and elsewhere in Europe. The discovery of uranium deposits near the town of Oklo, Gabon, was exciting news, but no one, at least initially, fully understood what had been discovered. That's how Perrin analyzed this strange sample. He and his colleagues confirmed that it was a natural sample that had undergone fusion when Earth was still a young planet.
Man-made nuclear fission reactors work by carefully controlling a chain reaction in which uranium-235 atoms are split by neutrons, releasing energy and more neutrons, which then go on to split other atoms. To sustain this reaction, "enriched uranium" is used, which increases the concentration of uranium-235. Creating such reactors requires advanced technology, precision engineering, and meticulous safety protocols. It's not something you'd expect to just happen in nature.
Yet, billions of years ago at Oklo, nature had spontaneously provided the right combination of uranium concentration, water, and geological stability to sustain a controlled fission reaction.
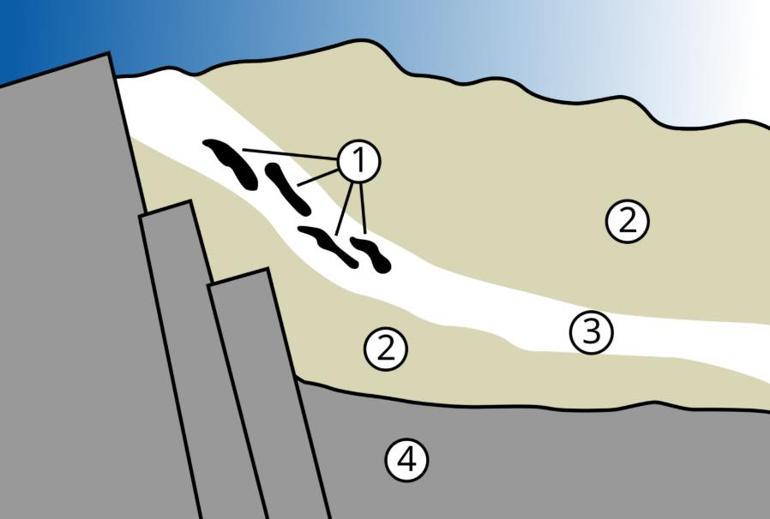
How to create a natural reactor?
In 1956, a chemist named Paul K. Kuroda predicted that natural fission reactors could form under the right conditions. His work attracted some attention, but it didn't immediately resonate because the conditions seemed unlikely and few (if any) people actually expected to find such a thing.
Kuroda estimated that to sustain a natural nuclear fission reaction, the uranium deposit would need to be at least 0.66 meters thick. If the deposit were smaller than this, it would not reach critical mass. The deposit also needed to contain sufficient uranium-235.
Two billion years ago, uranium-235 was much more abundant than it is today. At that time, it comprised about 3 percent of natural uranium, a level similar to the enriched uranium used in modern nuclear reactors. Just as in modern reactors, a "moderator" was needed to slow down the neutrons and make fission more likely. At Oklo, groundwater played this critical role. By slowing down the neutrons, the water facilitated a sustained chain reaction.
"Without anything to slow down the neutrons, to moderate them, as in a man-made light water nuclear reactor, the fission reactions simply stall. At Oklo, the water acted as a moderator, controlling the chain reaction by absorbing the neutrons," said Peter Woods, team leader responsible for uranium production at the International Atomic Energy Agency.
It also needed to be free of elements like boron or lithium, which absorb neutrons and stop fission. Fortunately, Oklo's deposits were devoid of such 'contaminants,' allowing the reaction to proceed. When these conditions came together perfectly, the result was a natural nuclear reactor .
The ancient reactor at Oklo didn't operate continuously. By dating rocks and analyzing past activity, researchers have found that the Oklo reactor operated in cycles.
As groundwater seeped into uranium deposits, it tempered the neutrons, allowing fission to occur. The reaction heated the water, eventually boiling it into steam. Without water to moderate the neutrons, the reaction stopped.
"That's what makes this so fascinating: that time, geology and water conditions combined to make this happen and to have it preserved to this day. The detective story has been successfully solved," Woods said.
Once the area cooled and more groundwater seeped in, the reaction would begin again. This cycle then repeated itself for hundreds of thousands of years. Research on the Oklo reactor concluded:
"About 15,000 megawatt-years of fission energy were produced over several hundred thousand years, equivalent to operating a large 1,500 MW reactor for ten years."

A natural area unlike any other
News of this natural phenomenon spread quickly. In 1975, physicists from around the world gathered in Libreville, Gabon, to discuss what became known as the Oklo Phenomenon. The discovery was striking. It revealed that nature had mastered nuclear power long before humans had even imagined it. Yet, while some theoretical predictions aligned with observations, proving what was happening proved difficult. There were four naturally occurring hotspots, all within the same geological structure.
The key to solving these puzzles lay in an unlikely source: xenon gas. Trapped within the minerals at Oklo, this inert (inactive) gas served as a time capsule.
Different xenon isotopes are formed during nuclear fission, and their ratios can reveal the conditions under which fission occurs. Physicist Alex P. Meshik analyzed these xenon isotopes and found that they hold clues to the reactor's stability. The trapped xenon suggested that the reactor's fission reactions were extremely stable, switching in and out as groundwater levels changed.
The xenon also revealed how the reactor eventually shut down: over time, the uranium-235 was gradually depleted, and the fuel supply fell below the critical threshold needed to sustain fission.
Although Oklo's uranium mines are now depleted, the legacy of the world's only known natural nuclear reactors lives on. Examples of Oklo reactors are preserved in museums like the Vienna Natural History Museum, where visitors can see the rocks produced by nature's fission reactor.
There may be other natural reactors, they just haven't been found yet.
milliyet





